Throughout Your Patient’s CPP Journey, Start and Stay on Triptodur
LH suppression at 1, 6, and 12 months1
Triptodur suppressed LH to prepubertal levels (GnRH-stimulated LH ≤5 IU/L) as early as month 1 in a clinical trial of treatment-naïve children

95% of children (42/44) achieved LH suppression with Triptodur at month 1

93% of children (41/44) achieved LH suppression with Triptodur at month 6 (primary endpoint)

98% of children (43/44) achieved LH suppression with Triptodur at month 12
Three patients had nonsuppressed LH levels at month 62
- Two of these nonresponders showed prepubertal LH levels at month 12, 1 of whom had a borderline LH value of 5.1 IU/L at month 6 but a suppressed testosterone level of 2 ng/dL
- The other encountered a technical problem with the first injection, which is likely to have played a significant role in this treatment failure
- The third nonresponder was a 9-year-old overweight boy with a BMI of 23.1 kg/m2, who may have required a higher drug dose for adequate hormonal suppression, or who may represent 1 of the rare children with CPP who do not achieve suppression with GnRH agonists


The power to pause with Triptodur
Triptodur arrested or reversed clinical signs of puberty in a clinical trial

95% of children (42/44) demonstrated a slowing of accelerated bone maturation at month 121,2

Sexual maturation (Tanner stage) was stable or reduced in 89% of children (39/44) at month 121,2
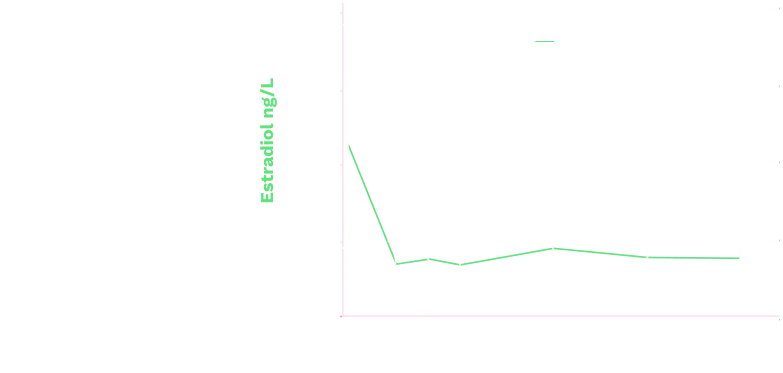
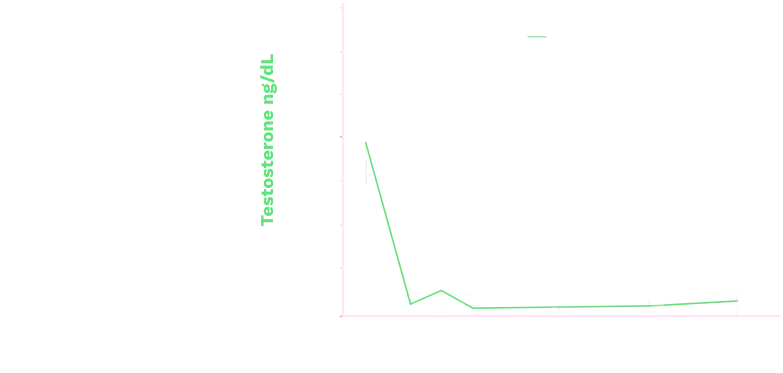
Triptodur effectively suppressed
sex steroids2
Mean (+SD) Serum Estradiol Levels in Girls (ITT Population)
Mean (+SD) Serum Testosterone Levels in Boys (ITT Population)
Abbreviation: ITT=intent-to-treat.

Established Safety and Tolerability
In a clinical trial, Triptodur was shown to be well tolerated1,2
No treatment discontinuations in a clinical trial2
All patients completed 48 weeks of treatment with Triptodur, and there were no treatment interruptions
Stable laboratory parameters2
There were no substantial, unexpected, or clinically significant changes in laboratory parameters or vital signs in children receiving Triptodur

Injection-site tolerance rated as very good2
Local tolerance at the injection site was judged to be very good, both immediately after and 2 hours after the first and second injections of Triptodur
Transient injection site reactions
Injection site reactions occurring in patients immediately and/or 2 hours after injection included1
- Pain (45%)
- Redness (14%)
- Pruritus (2.3%)
- Swelling (2.3%)
Only 5 adverse events (AEs), reported in 4 patients, were considered as triptorelin-related in the clinical trial, including vaginal or menstrual bleeding in 3 girls.
| Adverse Reactions1 | Number of Patients Reporting Event n (%) (N=44) |
|---|---|
| Infections and Infestations | |
| Nasopharyngitis | 6 (13.6) |
| Upper Respiratory Tract Infection | 4 (9.1) |
| Gastroenteritis | 3 (6.8) |
| Bronchitis | 2 (4.5) |
| Otitis Externa | 2 (4.5) |
| Pharyngitis | 2 (4.5) |
| Sinusitis | 2 (4.5) |
| Influenza | 2 (4.5) |
| nervous system disorders | |
| Headache | 6 (13.6) |
| Reproductive System and Breast Disorders | |
| Menstrual (vaginal bleeding) | 3 (7.7) |
| Respiratory, Thoracic, and Mediastinal Disorder | |
| Cough | 3 (6.8) |
| Vascular Disorders | |
| Hot Flush | 2 (4.5) |


Psychiatric events have been reported in patients taking GnRH agonists. Postmarketing reports with this class of drugs include symptoms of emotional lability, such as crying, irritability, impatience, anger, and aggression. Monitor for development or worsening of psychiatric symptoms during treatment with Triptodur.1